|
Current research |
|
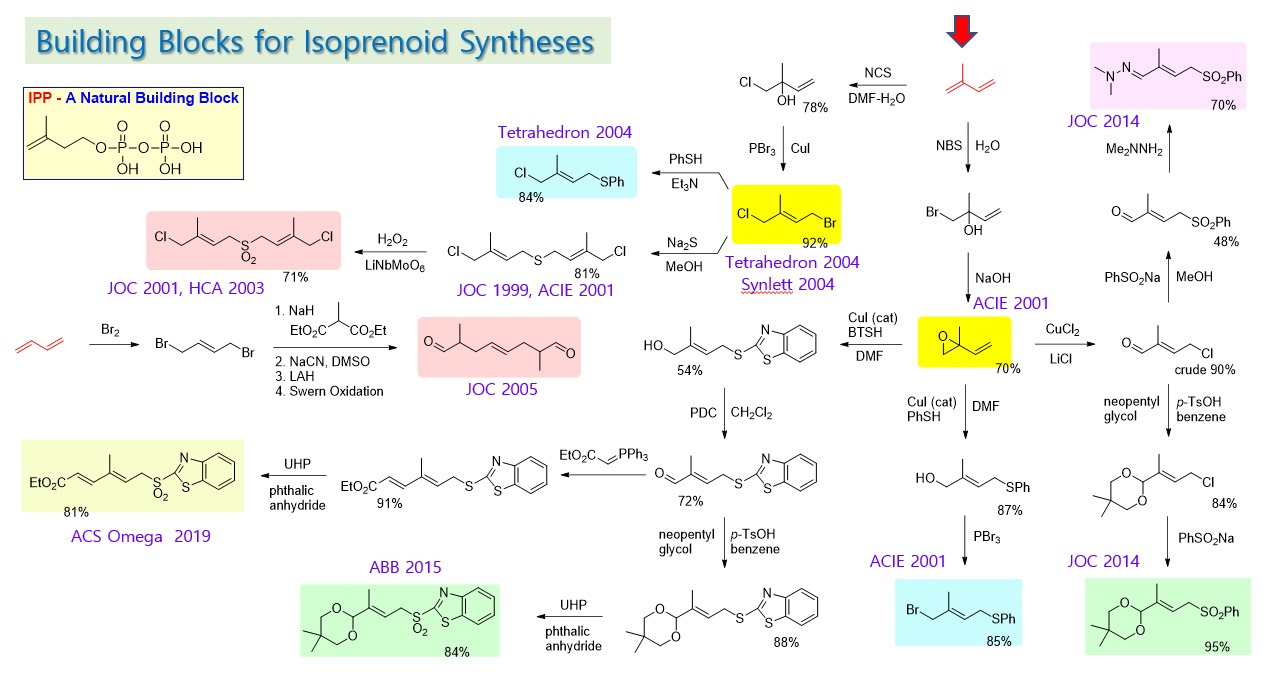
1. Development of functionalized building blocks for isoprenoid synthesis
Isoprenoids are physiologically important natural products, produced as secondary metabolites in vivo for antioxidant, immune function, self-protection, etc. IPP (Isopentenyl Pyrophosphate) is used as a basic building block in nature for tens of thousands of isoprenoid natural products. Chain extension of IPP, oxidation and cyclization of the extended chains are mediated by the action of various enzymes. Although target-oriented organic synthesis method may be useful for the preparation of the specific natural product, it is difficult to generalize and apply it to the synthesis of various related products. We adapted the bio-mimetic approach to generalize the synthesis of various isoprenoid natural products. Novel basic building blocks have been designed to secure the selectivity of reactions by tuning various functional groups. We developed methods to introduce two different functional groups selectively from isoprene (C5) as a starting material. We were able to induce directivity and selectivity of the chain extension reaction utilizing steric and electronic elements. Particularly, a site-selective ring opening reaction of an allyl epoxide using a copper catalyst and a thiol nucleophile in a DMF solvent was developed to efficiently manufacture building blocks for the chain extension. On the other hand, C10 units necessary for efficiently synthesizing the carotene structures were developed as a method of utilizing symmetry and a method of simultaneously forming four double bonds in a single reaction.
|
|
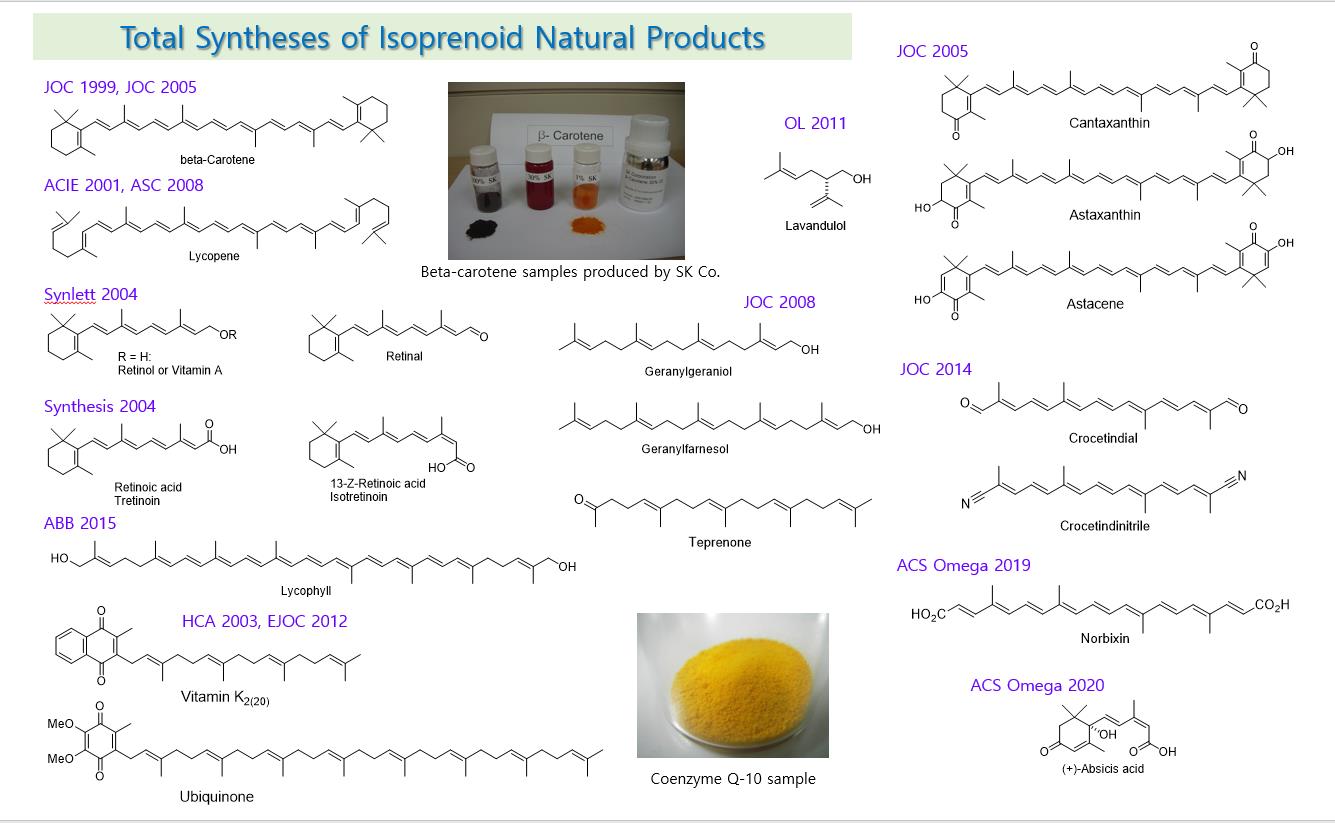
2. Development of synthetic method of bioactive isoprenoid natural products by chain expansion of the novel building blocks
|
|
|
|
Isoprenoids that are biosynthesized from IPP are classified into carotenes and terpenes according to their structural similarity. Carotenes have a structure of conjugated polyene chains, and terpenes have a structure in which carbon-carbon double bonds and single bonds are alternated. The basic skeletons were synthesized through the chain extension reaction of common building blocks mediated by a sulfone group. An elimination pattern of a sulfone group differentiates the final natural products, that is, a sulfone group is reduced by a boron reagent, LiBHEt3 in a palladium catalyst to prepare a terpene compound. And, using a base such as KOMe to form a double bond led to a carotene compound. Carotene and terpene compounds may contain ring structures and various oxygen functional groups at the ends of the chain, and for their synthesis, building blocks containing ring structures or oxygen functional groups have also been developed. Carotene compounds with a polyene chain structure are heat- and light-sensitive, and their syntheses require special cautions, especially because they are easily deteriorated under acidic conditions. Therefore, after the chain-extension for the necessary skeleton, several double bonds were formed simultaneously in the final step, thereby an efficient method of manufacturing the polyene chain was developed. Coenzyme Q-10 was a natural product with very high industrial demand at the time when developed in 2002. The attachment of decapreniol (C50) to the hydroquinone nucleus by Friedel-Craft reaction raised a serious side reaction problem of cyclization. After first attaching a C5 sulfone building block, and then combining it with solanesol (C45), extracted from tobacco leaves, it removed the sulfone group to complete the total synthesis of coenzyme Q-10 with practicality and efficiency.
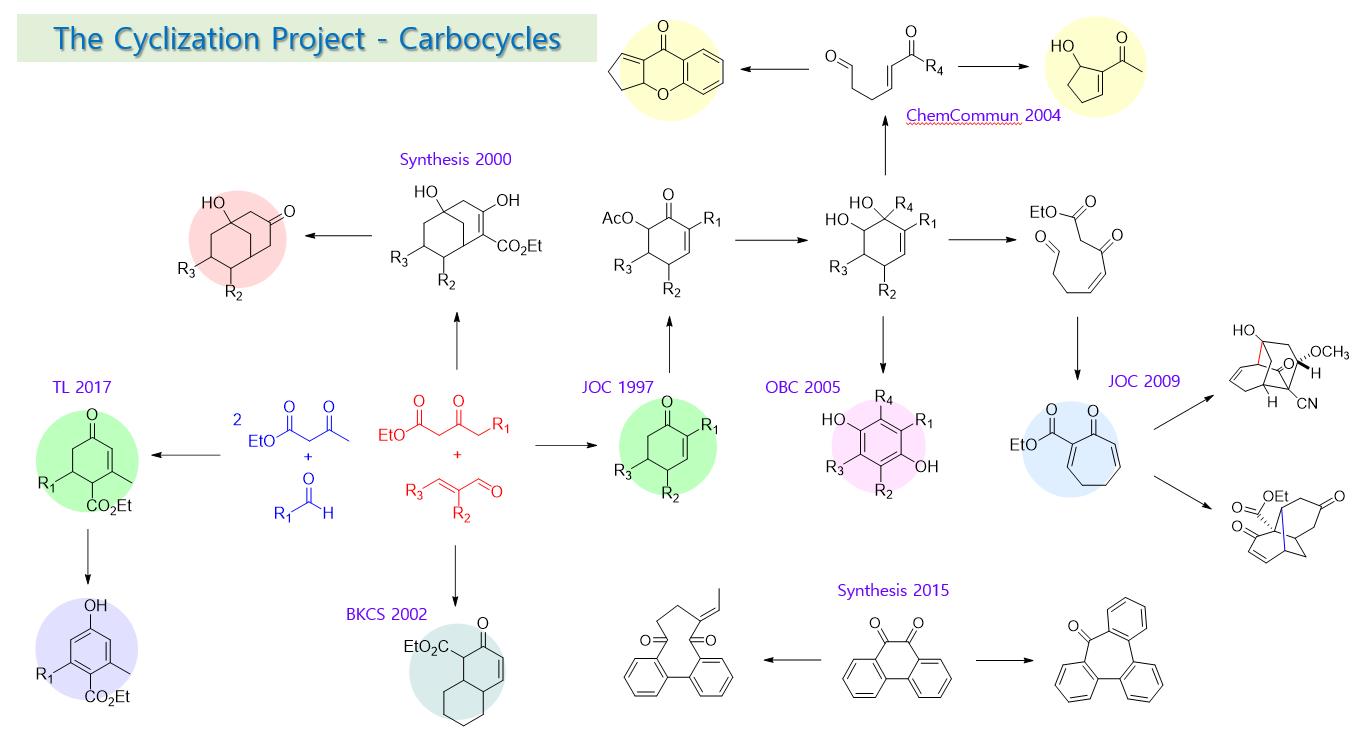
3. Preparation of useful carbocyclic compounds from simple chain compounds
|
In addition to the research on chain extension, the research on ring formation was also conducted. Carbo-cyclic compounds containing various functional groups, such as Hagemann's ester, often exhibit biological activity and are used as pesticides and antibacterial agents. Starting with the development of a synthesis method for cyclohexenones containing various substituents using ethyl acetoacetate and conjugate aldehyde, a simple chain material, a method for synthesizing bicyclo[3.3.1]nonane structure by double addition of ethyl acetoacetate was then developed. Meanwhile, the synthesis of decalone required for steroid synthesis was also performed.
In general, 6-membered ring compounds are structurally stable and can be obtained relatively easily compared to the other size rings. Thus, methods for efficiently synthesizing 5-membered and 7-membered ring compounds were developed by forming a diol compound from cyclohexenone and inducing ring contraction or expansion during the re-cyclization reaction of the chain compound prepared by its ring opening reaction. Since the cyclic compounds thus formed contain various functional groups, additional cyclization reactions are possible to provide chromone or caged compounds. Recently, a method of easily preparing Hagemann-type ester by reacting 2 equivalents of ethyl acetoacetate with a simple chain form of aldehyde and a method of synthesizing 4-hydroxybenzoic ester by simple oxidation reaction were developed. It can provide a carotene compound containing a phenol substituent with excellent antioxidant ability.
4. Acetoacetate protocol – cyclization to hetero-aromatic compounds by radical oxidation
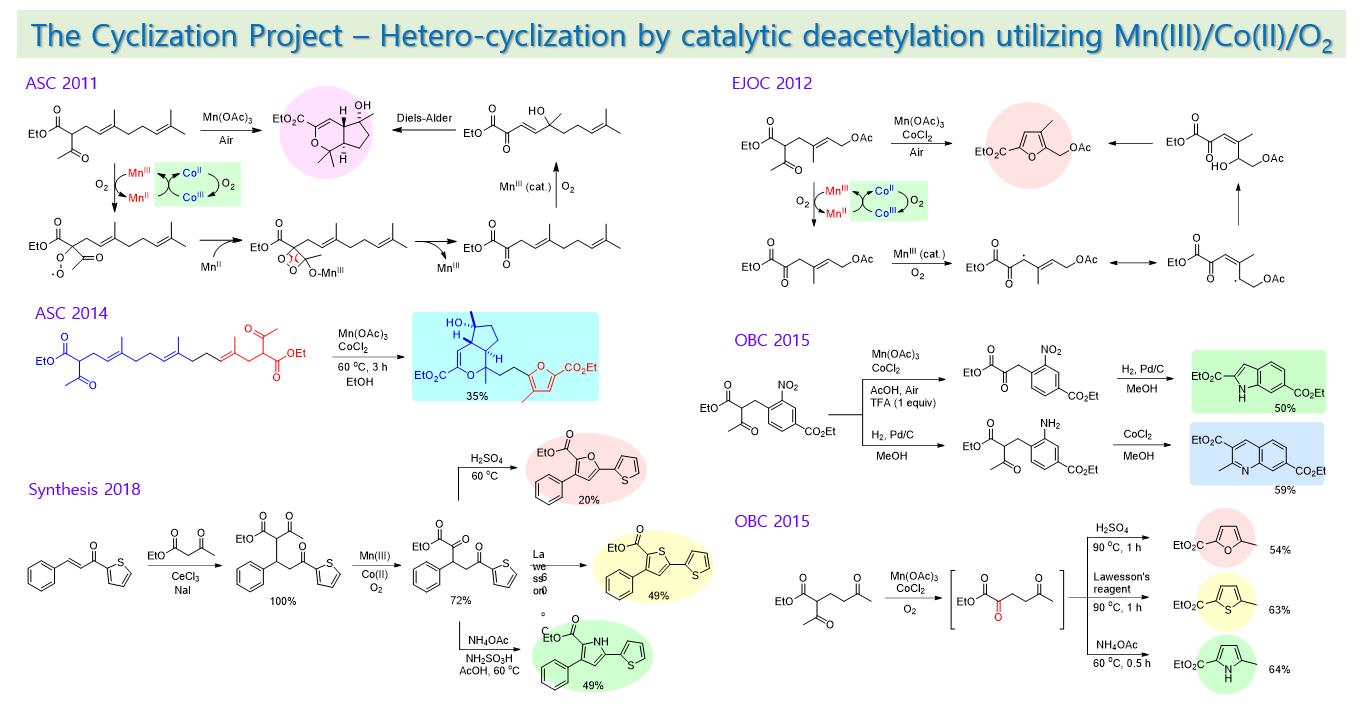
Acetoacetate compounds can be used to form carbon-carbon bonds efficiently by forming stable anions. After that, instead of proceeding with the decarboalkoxylation reaction, it was found that a deacetylation reaction can be induced by a radical oxidation reaction using a manganese(III)/cobalt(II) catalyst in the atmosphere. After performing a radical oxidation reaction by a manganese catalyst using a compound in which acetoacetate is attached to the terpene chain, a bicyclic pyran compound through hetero Diels-Alder reaction or a furan compound by cyclization was prepared according to the position of the methyl substituent. In the compound containing acetoacetate at the ortho-benzyl position of nitrobenzene, the order of the oxidative deacetylation reaction using manganese and the reduction reaction of the nitro group to amine was decisive in controlling the method of providing indole or quinoline.
On the other hand, ethyl acetoacetate undergoes 1,4-addition to methyl vinyl ketone to provide a 1,5-dicarbonyl compound. A 1,4-dicarbonyl compound is obtained through a deacetylation reaction of a manganese catalyst. It provides furan, thiophene, and pyrrole compounds by the Paal-Knorr reaction. The above method can be utilized in the development of new drugs for antibacterial, anti-cancer, and pain-relieving by providing various heterocyclic compounds. In addition, the furan and thiophene compounds containing an acetyl group at the 2-position provide a chalcone-like compound by aldol condensation with benzaldehyde. The 1,4-addition of ethyl acetoacetate, deacetylation by a manganese catalyst, and Paal-Knorr reaction produced bichalcopene compounds, which can be used as an organic electronic material as well as an anti-bacterial agent.
5.. Research in progress (biomass conversion, lithium electrolyte additives, organic molecular conductors, etc.)
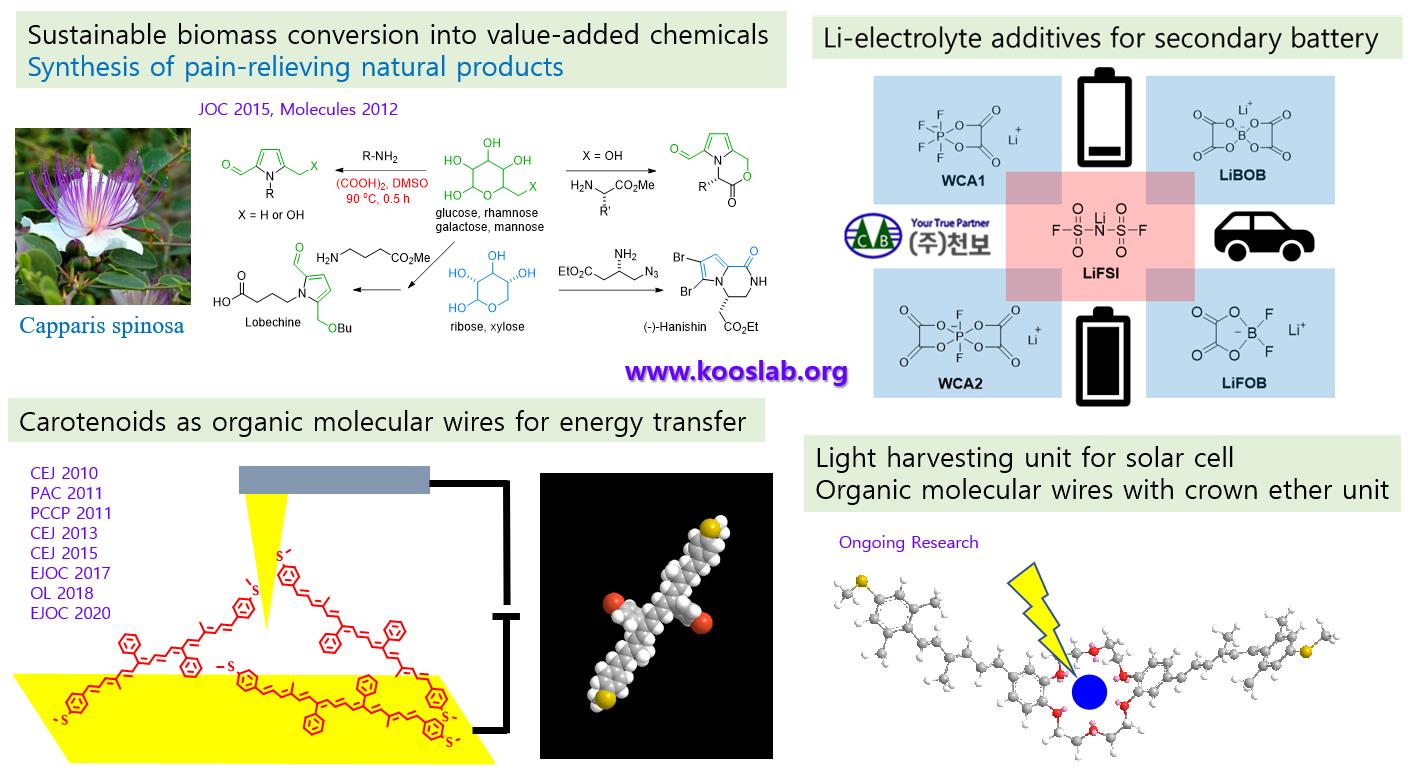
Since 2011, research has been conducted to secure future energy sources due to depletion of petroleum resources. Study on the synthesis of 5-hydroxymethylfurfural (5-HMF), which can be used as an energy substance and a platform chemical from sustainable reducing sugars, has been carried out. Modified Maillard reaction between glucose and amino acids produced a pyrrolo-lactone compound, a pain-relieving folk-medicine, in one-pot reaction. The reaction between amino acid and ribose produced pyrraline compounds, novel platform chemicals which may find a way to develop new drugs for antibacterial, anti-cancer and pain-relieving effects through their additional cyclization reactions.
In addition to their antioxidant functions, carotene compounds are known to play an important role in energy transfer in photosynthesis of plants or plankton. The structure of carotene compounds was modified to secure stability as an electronic material, and thereby to transmit and to control energy more efficiently. Research was conducted to produce carotenoid organic molecular wires with variable conductance. It was proved that conductivity of 100 times or more can be controlled according to the type of phenyl substituent in the carotene compounds with the same length. In addition, a carotene molecular sensor in which crown ether is introduced between carotene polyene structures is being developed, and it has been confirmed that each has different electrical conductivity values depending on the type of bounded metal ions. Development of a light-harvesting unit in solar cell using the photoelectric effect of metal ions and the energy transfer effect of a carotene molecular conductor is also underway.
On the other hand, since 2012, through the industry-academic cooperation study with Cheonbo Co., Ltd., we have developed an optimized production method of LiFOB, LiBOB, WCA1, WCA2, and LiFSI, lithium electrolyte additives in the secondary battery.